
Contribution to the good health of people
When JPS Pharmaceutical produces kampo medicine, centrifuges are employed in the process of solid-liquid separation to split crude drugs and extract liquid. By using centrifugal force, the liquid can be taken out without squeezing the crude drugs too hard. Additionally, we take a very short time and use low temperatures to make the liquid concentrated, so that we produce bright-colored extract without losing its fragrance as if it were brewed in an earthen teapot. Besides providing such quality kampo in a stable manner, we are spreading the idea of self-prevention and self-medication and proposing lifestyles that are in harmony with nature, thereby helping people improve their health.

Integrated manufacturing in Japan
Our strength is that we can manufacture high-quality Kampo and crude drug preparations through integrated domestic manufacturing at our own factories, from the management of crude drugs to the production and packaging of extracts.
Carefully selected crude drugs
The raw materials and crude drugs for extracts, which greatly affect quality, are rigorously checked for each crude drugs, and purchased in a form as close to their original form as possible.
Commitment to water
We purify water at our own factories and always use clean water of constant quality for extract extraction. Wastewater is also purified with the help of microorganisms.
JPS Extract Logo Mark
Extracts considerably influence the quality of kampo products. We produce extracts, which are the core of our quality, with great care from scratch, having kept the obsession with extracts since our founding. The JPS Extract Logo Mark proves “JPS Pharmaceutical uses kampo extracts that are manufactured in its plant in Tochigi Prefecture; it does so based on the strict quality policy of JPS, the slogan of which is “JPS Pharmaceutical contributes to people’s healthy and affluent lives and the future of medicine” by offering pharmaceutical products.
Employees with this insignia attached to their jacket have received JPS employee training and work hard every day to provide accurate information on pharmaceutical products.
Formulating a BCP
A Business Continuity Plan (BCP) is a plan designed to prevent a company from going bankrupt or downsizing after the occurrence of an unforeseen event, such as a natural disaster or a pandemic of infectious diseases, ensuring that business can continue.
As a pharmaceutical company, providing a stable supply of medicinal products is one of our primary missions.
Shrinkage in business will also impact our ability to employ staff.
The company’s BCP was thus developed to protect both people and business from such emergencies.

A crude drugs warehouse in the aftermath of the Great East Japan Earthquake
Protecting the Lives of Employees
To prevent damage from falling objects or overturned equipment, we took measures such as fixing in place all the shelves at our head office, factories and local liaison offices. We also reformulated our disaster prevention manual to ensure that appropriate action can be taken in the event of a disaster.

Plant disaster drill (August 2022)
Rebuilding on Our Own
As a company, JPS Pharmaceuticals aims to be a provider of support, rather than a recipient. In order to do that, we must maintain the ability to provide rescue, first aid, food and other care on our own. Each of our sites is thus equipped with stockpiles and rescue equipment.
The Tochigi Plant also conducts "blackout start" drills to ensure that power can be generated without an external power source in the event of a power outage.

Stockpiles at Headquarters (only a portion)
Supporting the Community
Our Tochigi Plant shares a disaster support agreement with the town of Haga and Moka City. In the event of a disaster, our facilities can be used as a free charging station.

The Tochigi Plant cogeneration system
Our BCP will continue to be reviewed and revised on a regular basis to ensure that we are fully prepared for business continuity.
Contribution to local communities
Agreement on the supply of OTC pharmaceutical products in the event of disasters
One of our partnerships is the Agreement on the supply of over-the-counter (OTC) pharmaceutical products in the event of disasters. The agreement is part of our partnerships with Hagamachi (a town) and Moka-shi (a city) in Tochigi Prefecture, where our plant is located, to ensure the smooth provision of pharmaceuticals in case of disasters. The plant has adopted an energy supply system and has an agreement with Hagamachi to offer the use of the plant as a venue for charging mobile phones and other devices for free during disasters.
Social Studies Field Trip for Elementary School
As part of a social studies field trip for a nearby elementary school, students visited the JPS kampo lism store. The students had the opportunity to learn about kampo medicine by holding and smelling the crude drugs and experiencing grinding with special tools "Yagen".

Exhibited at Sou Terrace Festa
We exhibited at a community event "Imoni Kai & Sou Terrace Festa" held at the Municipal Archaeological Center in Yokohama. We enjoyed interacting with the local community and visitors to the event had the opportunity to learn about herbal medicine through a Yagen experience and other crude drug displays.

Preservation of the consistency of the Earth
Utilization of crude drug residues
A pulpy matter made from crude drugs is generated in the production process of kampo medicine, and we offer it to farmers near the plant to use as an ingredient of fertilizers or composts. Two to four 2-ton truckloads of crude drug residues are generated a day, depending on the daily output. At present, the recycling rate of crude drug residues is 100%, but we have to keep looking for users in order to maintain their stable reuse.
Introduction of users of crude drug residues
1. Big Dream Oshima Bokujo (ranch)
Big Dream Oshima Bokujo, situated in Hagamachi, Tochigi Prefecture, raises about 60 cows. Mixing our crude drug residues with cow manure, rice husks, etc. to produce compost, the ranch uses it as a fertilizer for their grass. Milk from the cows there is very rich.
This process establishes an almost perfect recycling system from cow to manure to compost to grass.

Healthy cows raised with grass fertilized by crude drug residues
2. The Kobanawa farming family
A married couple is engaged in farming. They mainly grow rice but also produce asparagus. Although it is said that the root of an ordinary asparagus plant can last about eight years, some asparagus plants with 17-year-old roots are thriving here.

Fully grown asparagus
3. Employee Home Gardens
Crude drug residues is also used as fertilizer in the home gardens of our company’s employees.
Fertilizer with crude drug residues can help soil maintain good water retention and breathability, enabling vegetables to be grown with less fertilizer used overall.
The vegetables that result have deep roots and a reputation for being very delicious.
Some of the vegetables grown include tomatoes, eggplants, Chinese cabbage, radishes and rice.

Chinese cabbage

Eggplants

Rice
Wastewater treatment using microorganisms
Wastewater from the plant is collected in the wastewater treatment facility. The water is treated by aerobic bacteria, such as Pseudomonas and Bacillus, that absorb and decompose organic substances in the wastewater. In order to make these microorganisms work efficiently, we control the pH of the wastewater and the amount of organic matter flowing into the reaction tank and manage the oxygen concentration, turbidity, chromaticity and other properties. We also deploy sand filters and activated carbon adsorption towers to discharge the treated water. This effort can stop the water quality from deteriorating by preventing wastewater containing organic substances from flowing or leaking into rivers and soil.

Wastewater before purification
All the wastewater from the plant, such as water used for cleaning machines, wastewater from cooking or washing, and sewage, is turned into clean water before being discharged.

During purification
A wastewater treatment facility called an aeration tank uses microorganisms to decompose and purify pollutants in wastewater. The water becomes murky temporarily during purification.

Discharged water after purification
In order to discharge used water, the water has to be purified through 10 processes or more and further, satisfy strict standards.
Management items cover diverse fields, including daily management items, bi-daily management items, and weekly management items, and reporting is done monthly. On top of this, we commission specialist dealers to perform management and inspections on a regular basis.

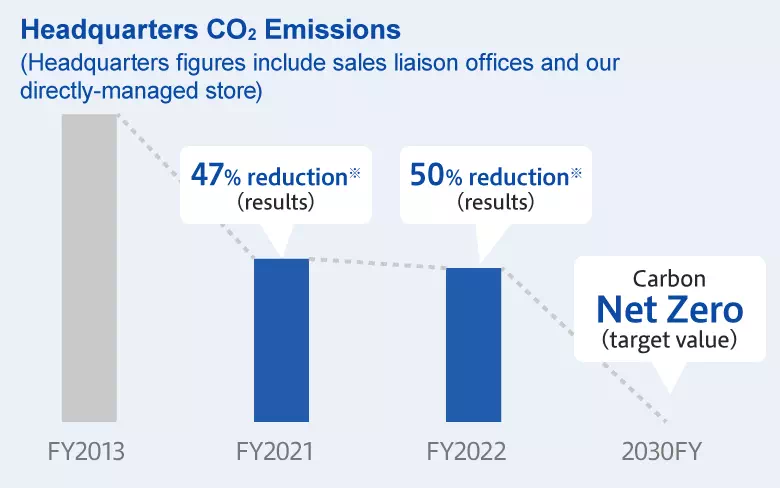
CO2 Reduction Plan
Aiming for carbon neutrality by 2050, the Federation of Pharmaceutical Manufacturers' Association of Japan has announced a revision to its target that set “Net Zero CO2 Emissions by 2050” as its long-term vision and a “46% reduction of CO2 emissions in FY2030 from the levels of FY2013” as a Phase II Target. JPS Pharmaceutical has set its targets so as to achieve Phase II.
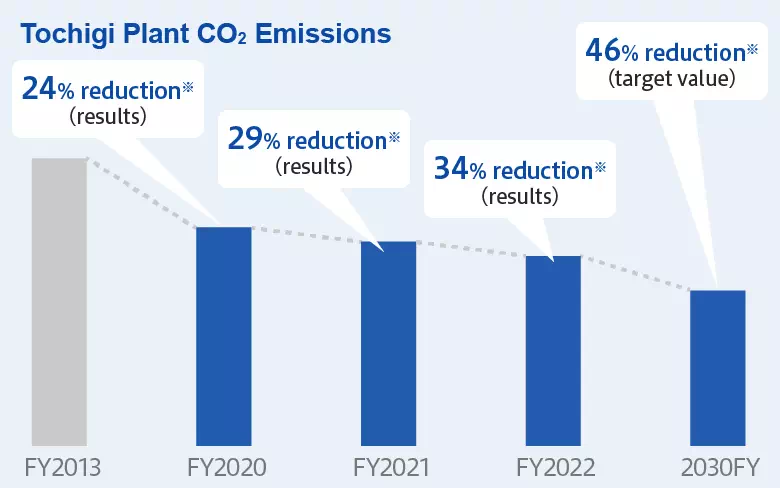
*from the level of FY2013

Energy supply system
Cogeneration system
*from the level of FY2013

Introduced electric vehicles
2020:
Achieved about 7% reduction in CO2 emissions, due to the introduction of an energy supply system at our Tochigi Plant
2021:
Implement target of 46% reduction from FY 2013 levels by FY 2030
*Both headquarters and plant figures apply only to scope 1 and scope 2 emissions
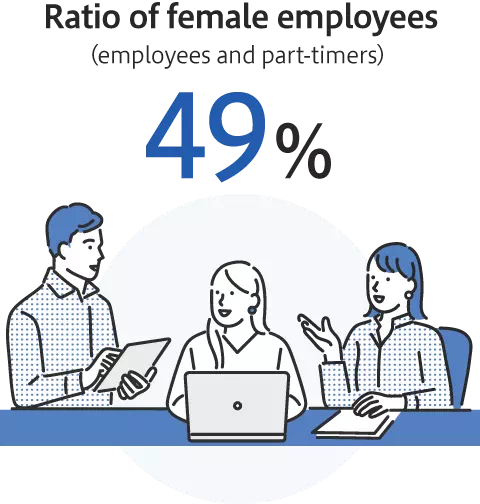
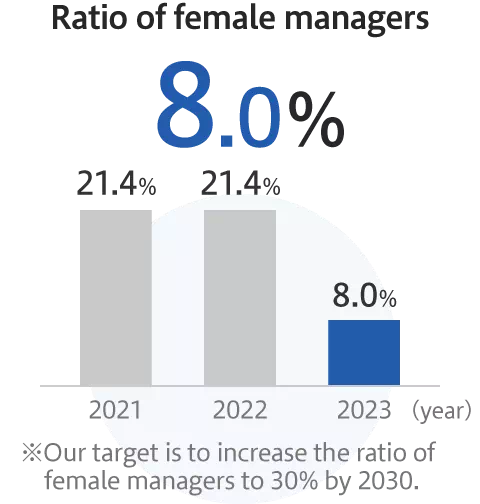
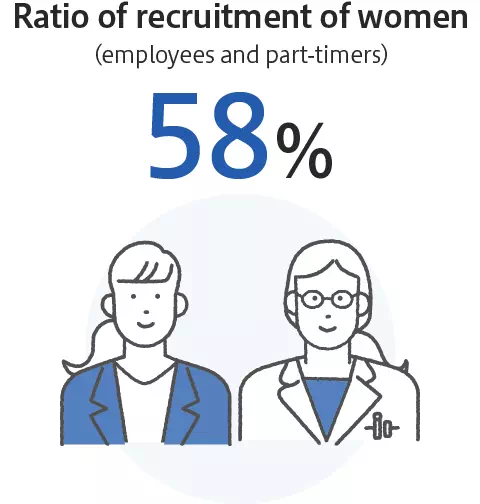
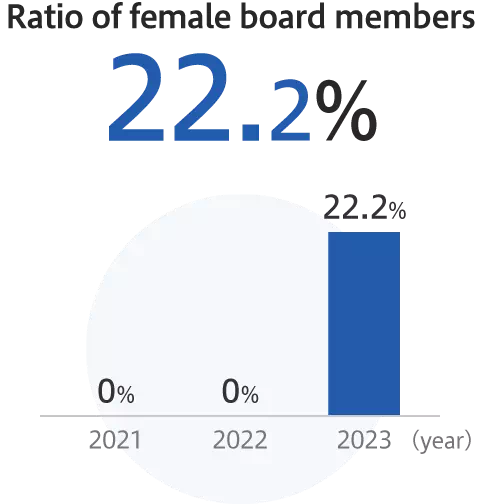
Promotion of diversity and inclusion
Enhancement of employees’ satisfaction and wellbeing
*As of January 2024


External evaluation
- 4 / 2024 Upgraded to Superior in the Y-SDGs, the SDGs Certification System of Yokohama City.
- 6 / 2023 We received financial support from Mizuho Bank to proceed with our SDGs.
- 3 / 2022 Received certification of Y-SDGs, the SDGs Certification System of Yokohama City.
- 1 / 2022 Announced registration with the Tochigi SDGs Promotion Company System.
- 25 / 6 / 2022 Received an SDGs promotion loan from Sumitomo Mitsui Banking Corporation.



