Integrated manufacturing in Japan

Manufacture in our own factory, so it is integrated and consistent
JPS Pharmaceutical is placing a greater emphasis on integrated manufacturing in our own factory under strict quality control. Flavors, colors, and effects close to original decoctions can be reproduced because we remain faithful to integrated manufacturing in Japan. You can see part of the manufacturing process in the factory below.
Manufacturing flow in JPS factory

Procurement of whole crude drugs
Whole crude drugs collected to the factory from designated production areas are stored in a temperature- and humidity-controlled environment.
Whole crude drugs are laid in stock as close to the original shape as possible for storage. This is because it is difficult to check quality in a shredded condition.

Cut up and blend whole crude drugs
To always keep the extract content consistent, the characteristic constituent for quality control contained in the crude drug is analyzed by liquid chromatography.
Since crude drugs are natural, the ingredient content is not consistent. JPS Pharmaceutical controls crude drugs’ characteristic constituents for quality control to stabilize ingredient content.
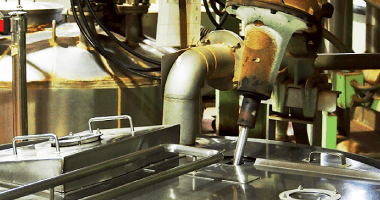
Extraction and separation
The same method as for decoctions is used for extraction. Extraction is performed using stable water which is produced by filtration through high-performance filter and reverse osmosis (RO) membrane.
Extraction is always performed in specified conditions. Only centrifuged supernatants are used as extracted solution.

Extract concentration and drying
Derived extract is concentrated and dried.
Prolonged concentration at high temperature can cause oxidization and degradation of crude drug extract solution by heat. However, extract quality can be protected by short-time concentration at low temperature.

Preparation and packaging
Dry extract is processed into forms such as tablets, granule, and liquid medicines and packaged.
Product design ranges from the cutting phase of crude drugs to preparation in order to avoid deterioration of quality after release and to facilitate quick dissolution in the body.

Release
Once products have passed the final test, they are released and delivered to customers via stores.